Purpose
In this experiment, we were trying to find out how yeast cells manage to communicate and thus mate asexually in order to produce offspring. In order for a message to be communicated, a signal must be sent and then received by another being; this lab will prove how yeast cells go through this communication process in order to produce a required response for the production of yeast.
Intro
Both unicellular and multicellular organisms use cell communication in order to elicit a response which helps an organism coordinate and respond to their environment. Cellular communication can occur through direct contact, local signaling, or long-distance signaling. For a response to occur, a message much reach a receptor that is able to receive that type of message which then turns on the ability for many other reactions, or a cascade. Pheromones are a type of signaling molecule that is secreted from one organism and detected from another organism thus providing various messages through this cellular communication and eliciting a change.
As you can see above, the budding and single cells are both present on this slide. Budding cells are cells clumped together, while single cells are the ones all by themselves. Budding cells contain both a and alpha DNA, while single cells contain either one or the other.
Methods
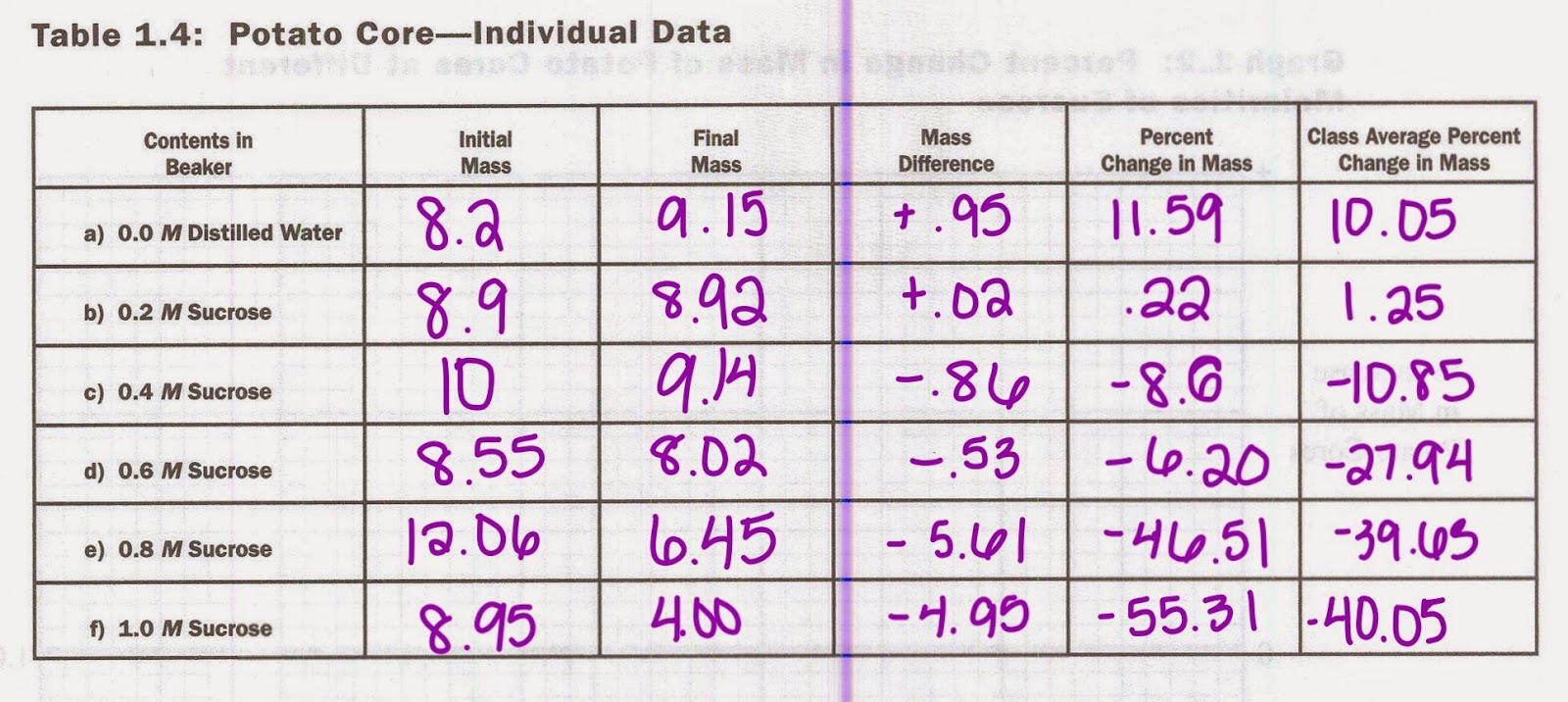
In our experiment, we tested yeast reproduction. We grew yeast is two different environments: broth and a sugar like gelatin. We created three copies of each environment. One with only a type, one with only alpha type, and one with both. We then took samples and placed them under a microscope.
We then counted how many yeast were present on a sample size of our tray and recorded it into our data.
Data
Data was gathered by the picture taken through the microscope during the lab and counting out the cells found within that field
This table shows the number of single haploid and budding haploid cells within the alpha-type and a-type slides. As time increased, the number of budding haploids increased more than the single haploid cells.
This is a similar chart except this one contains the the number of various cells and zygotes from the mixed culture. The budding haploid, shmoos, budding zygotes, and asci all increased after 24 hours in the incubator.
Graphs and Charts
This graph reflects the first table of the a-type and alpha-type cells
This graphs reflects the second table above of the mixed combination of a-type and alpha-type cells
Discussion
Throughout this lab, there were a few notable differences between the A-type and Alpha-type strands of yeast while they were observed in their cultures. Over the course of the first night when stored in the incubator, the Alpha-type reproduced at a more accelerated rate than its counterpart. In addition to this, the genomes differed between both strands, yet under the microscope the behavior of each was nearly identical. In the isolated cultures, neither strand seemed to move, and no schmooz were formed. However, in the mixed culture, the pheromones from each strand were received by receptors within the plasma membrane of the opposite strand, and each strand morphed its physical structures to as to move closer to its counterpart. Schmooz can be detected by identifiying unique, irregular shapes of yeast when they are normally spherical, perfect circles.
It has become evident from this lab that yeast cells cannot communicate by direct contact, but strictly via pheromones. Yeast has no flagella or cilia to move, which is why schooz must be formed to acheive gradual movement across the dish. Pheromones are used to notify far-off yeast cells that the counterpart is present, so that they can reach each other and eventually mate. There is really no other means to communicate that other cells are nearby. Pheromones will be transduced faster if the yeast cells are closer together. For this reason, it is understandable if yeast cells that are further away take more time to respond by creating schmooz.
Conclusion
We can conclude from this lab that the most favorable environment for yeast reproduction is when there is ample food and both a type and alpha type present. This makes sense, as this is a very secure environment in which yeast can thrive. Although yeast can reproduce when there is only one reproductive type present, best results are achieved when their is a mix. It is always better to recombine DNA and increase genetic variability than to have many others have the same DNA. It leads to a higher survival rate for the species, because one genetic defect cannot wipe out the entire species.
References
http://rsob.royalsocietypublishing.org/content/3/3/130008
Carolina Cell Communication for AP Biology